Packaging Components
It can be packaged in bottles or blisters.
stability
AdVersa® can be formulated to address stability problems associated with many unstable and/or low solubility active ingredients. IntelGenx uses a special process to stabilize the formulations.
AdVersa®: A New Direction
AdVersa® is at the forefront of buccal mucoadhesive hydrogel technology combining maximum drug delivery efficiency and a patient oriented product. It is formulated with patients in mind. AdVersa® offers a unique alternative that focuses on patient comfort and compliance. It offers rapid or slow releasing alternatives, perfect for acute or chronic indications.
“With AdVersa® in place, patients can eat, drink and carry on normal daily activities.”
AdVersa® can be customized to your needs.
Intellectual Property Status:
AdVersa® is at the forefront of buccal mucoadhesive hydrogel technology combining maximum drug delivery efficiency and a patient oriented product. It is formulated with patients in mind. AdVersa® offers a unique alternative that focuses on patient comfort and compliance. It offers rapid or slow releasing alternatives, perfect for acute or chronic indications.
| Component | Title | Filing date | Current status as of Jan. 2017 | Subject matter |
|---|
| US Patent No. 7,592,328 |
Natural cyclodextrin complexes |
2003/02/20 |
Issued |
Composition of Matter |
| US Patent No. 8,735,374 |
Oral mucoadhesive dosage form |
2010/07/15 |
Issued |
Formulation > Composition |
| CA Patent No. 2,476,834 |
Natural cyclodextrin complexes of cannabinoids |
2003/02/20 |
Issued |
Combination > Composition and Pharmacokinetic |
IntelGenx also has the registered trademark ADVERSA®. The trademark is currently registered in the US.
Market Opportunity
AdVersa® can be a vehicle for many different drugs that can be negatively affected when delivered via traditional drug delivery methods. It is ideal for:
- Avoiding a metabolic first pass effect
- Maximum delivery efficiency of expensive active ingredients
- Actives with poor water solubility
- Immediate release of the active
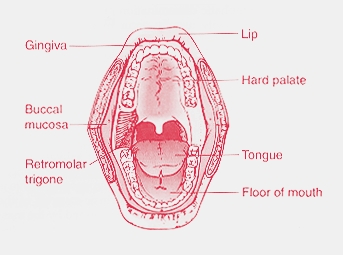
- Buccal or sublingual delivery
- Slow release of the drug
- Modular residential time
- Release of the drug without mastication or water